Navigation auf uzh.ch
Navigation auf uzh.ch
Neural stem cells (NSCs) generate new neurons throughout life (reviewed in: Denoth-Lippuner and Jessberger, 2021 Nature Reviews Neurosc). We use imaging-, genome editing-, and transgenesis-based approaches as well as cellular models of human diseases using pluripotent embryonic cells to study the molecular and cellular framework of NSC biology in the developing and adult brain. Aim of our research is to understand how physiologic and disease-associated alterations of neurogenesis are translated into stem cell-associated plastic changes in the developing and adult brain on a molecular, cellular, and behavioral level.
We pioneered intravital imaging of individual NSCs and their progeny in the mouse hippocampus and provided direct evidence for extended self-renewal of NSCs. Together with molecular tools (such as single cell RNA-sequencing), we currently use in vivo imaging to characterize the functional contribution of new vs. old granule cells for hippocampal circuit activity and how stem cell dynamics are affected by advancing age.
Recent publications
Bottes S*, Jaeger BN*, Pilz GA*, Jörg DJ, Cole JD, Kruse M, Harris L, Korobeynyk VI, Mallona I, Guillemot F, Helmchen F, Simons BD, Jessberger S (2021) Long-term self-renewing stem cells in the adult mouse hippocampus identified by intravital imaging. Nature Neuroscience 24:225-233
Pilz GA*, Bottes S*, Betizeau M, Jörg DJ, Carta S, Simons BD, Helmchen F, Jessberger S (2018) Live imaging of neurogenesis in the adult hippocampus. Science 359:658-662
UZH press releases:
Brain Stem Cells Divide over Months
Stem Cell Divisions in the Adult Brain Seen for the First Time
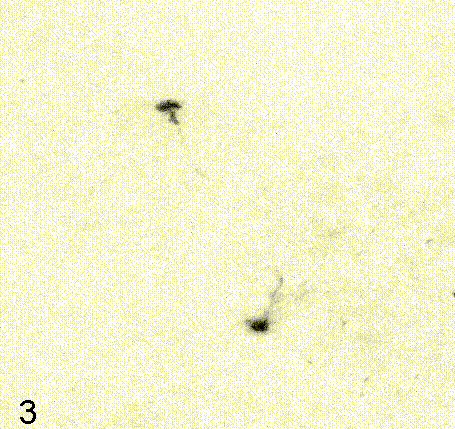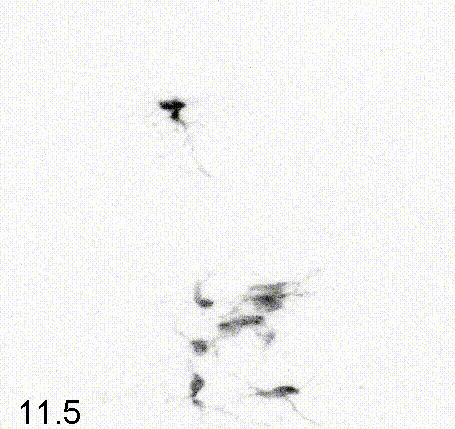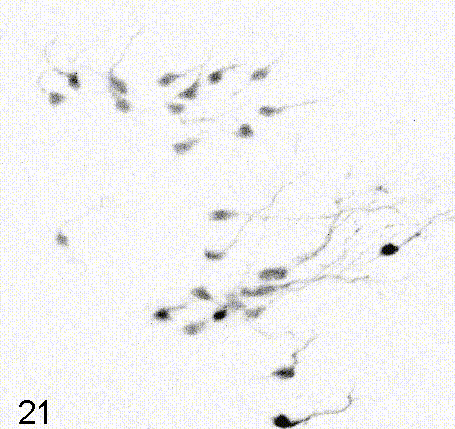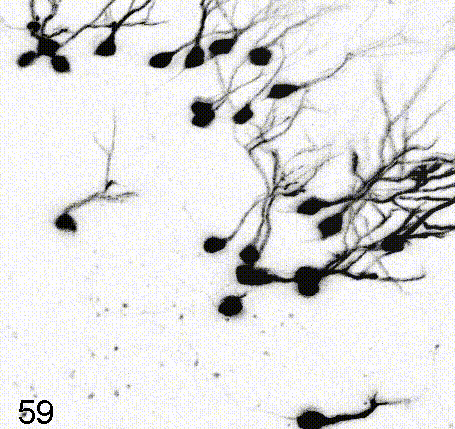
Intravital imaging of adult hippocampal neurogenesis over 59 days (e.g., Pilz et al., 2018 Science; Bottes et al., 2021 Nature Neuroscience). Credit: Gregor Pilz, HiFo, UZH
We provided the first description of a diffusion barrier in the endplasmic reticulum (ER) in mammalian cells that appears to be involved in the asymmetric segregation of cellular age. We currently aim to identify the molecular and behavioral consequences of previous cellular experiences (such as cell divisions and/or biological age) on somatic stem cells. We developed novel tools to identify the cell division history of stem cells in vivo (e.g., the iCOUNT system) and currently develop novel technology to visualize the cellular age of individual cells in complex tissues.
Recent publications
Denoth-Lippuner A, Jaeger BN, Liang T, Chie SE, Royall LN, Buthey K, Machado D, Korobeynyk VI, Kruse M, Munz CM, Gerbaulet A, Simons BD, Jessberger S (2021) Visualization of individual cell division history in complex tissues using iCOUNT. Cell Stem Cell 28:1-15
Bin Imtiaz MK, Jaeger BN, Bottes S, Machado RAC, Vidmar M, Moore DL, Jessberger S (2021) Decline of Lamin B1 expression mediate age-dependent decreases of hippocampal stem cell activity. Cell Stem Cell 18:S1934-5909
Moore DL, Pilz GA, Araúzo-Bravo MJ, Barral Y, Jessberger S (2015) A mechanism for the segregation of age in mammalian neural stem cells. Science 18;349
UZH press releases:
Reactivating Aging Stem Cells in the Brain
A barrier against brain stem cell aging
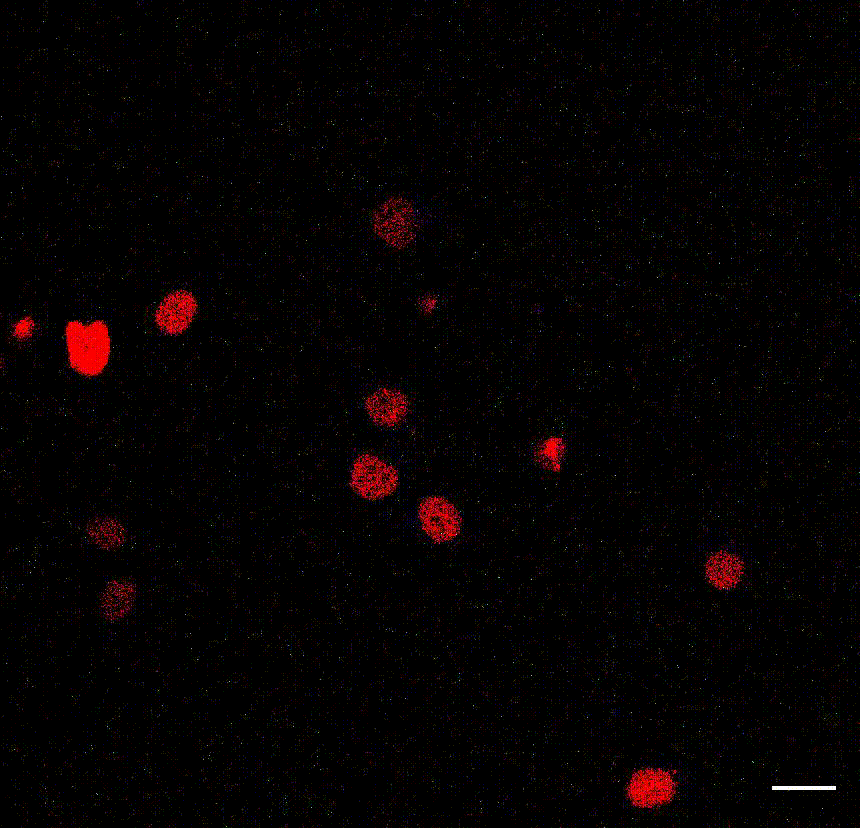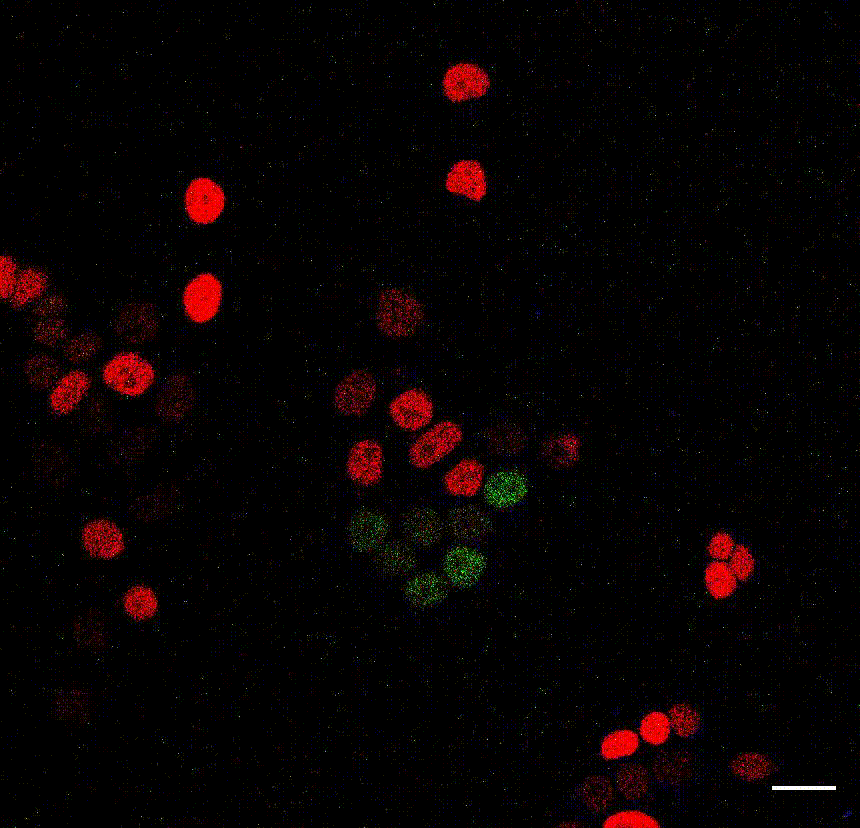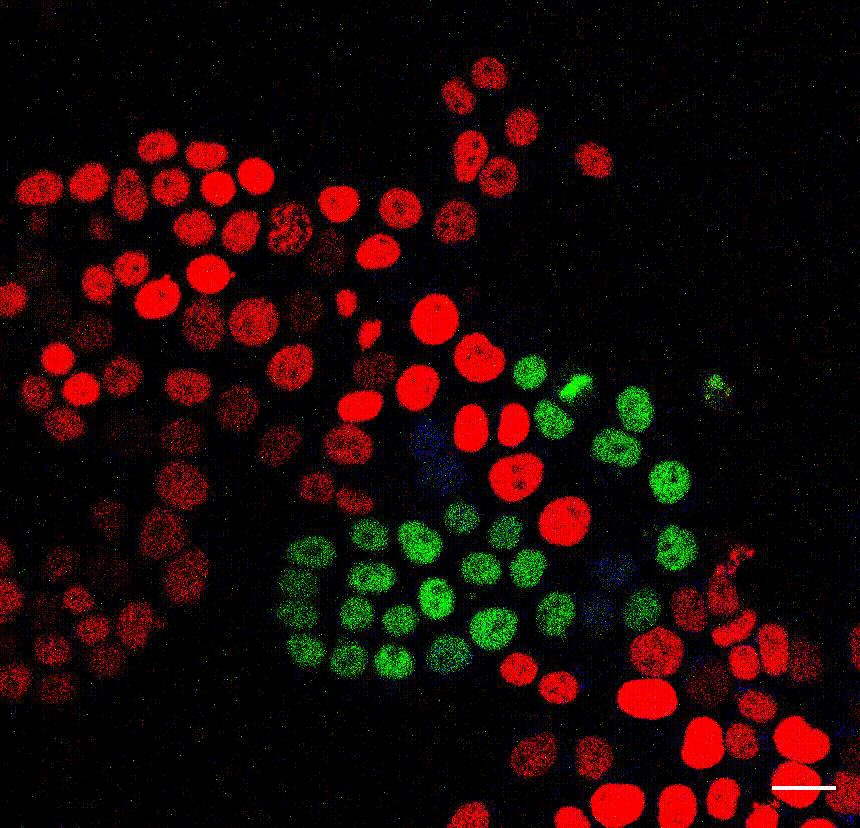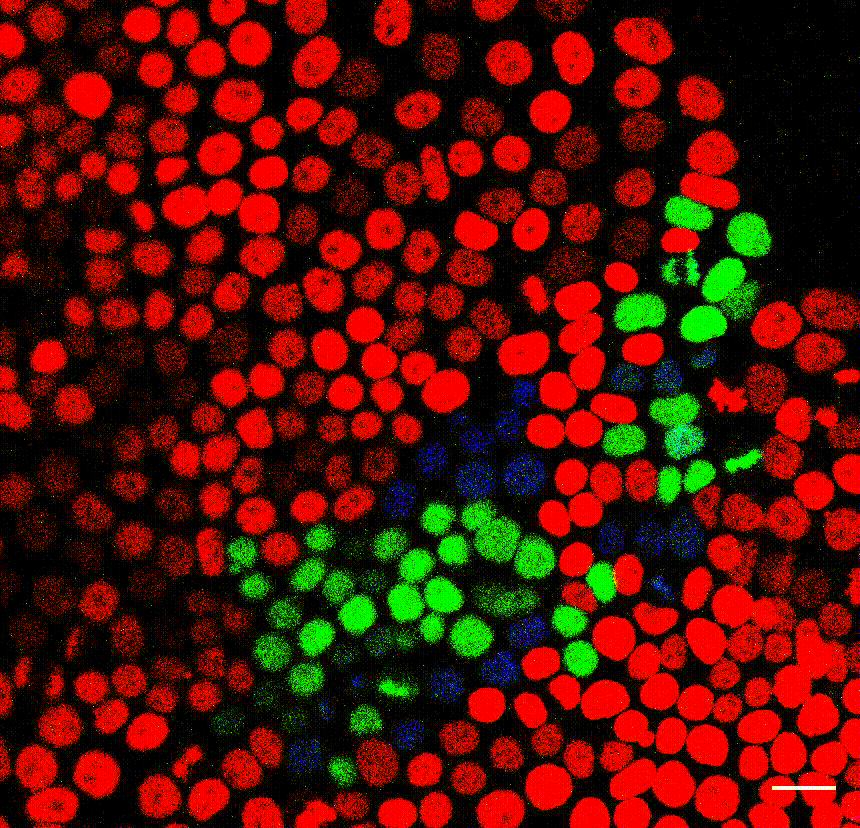
The cell division counter miCOUNT in embryonic stem cells (Denoth-Lippuner et al., 2021 Cell Stem Cell). Credit: Annina Denoth-Lippuner, HiFo, UZH
Our previous work identified several pathways/genes that are critically involved in distinct development steps during neurogenesis. We pioneered work to characterize a critical role for lipid metabolism for neural stem cell behavior. We currently use mouse genetics and regionalized organoids derived from human embryonic stem cells (hESCs) to characterize NSC behavior in complex tissues. Experiments are based on mouse genetics, hESC genome-editing, long-term imaging, and single cell RNA-sequencing approaches.
Recent publications
Bowers M*, Liang T*, Gonzalez-Bohorquez D*, Zocher S, Jaeger BN, Kovacs W, Röhrl C, Cramb K, Winterer J, Kruse M, Dimitrieva S, Overall RW, Wegleiter T, Najmabadi H, Semenkovich CF, Kempermann G, Földy C, Jessberger S (2020) FASN-dependent metabolism links neural stem/progenitor cell activity to learning and memory deficits. Cell Stem Cell 27:98-109
Wegleiter T*, Buthey K*, Gonzalez-Bohorquez D, Hruzova M, Bin Imtiaz MK, Abegg A, Mebert I, Molteni A, Kollegger D, Pelczar P, Jessberger S (2019) Palmitoylation of BMPR1a regulates neural stem cell fate. PNAS 51:25688-25696
Knobloch, M., Pilz, G.A., Ghesquiere, B., Kovacs, W.J., Wegleiter, T., Moore, D.L., Hruzova, M., Zamboni, N., Carmeliet, P., and Jessberger, S. (2017) A Fatty Acid Oxidation-Dependent Metabolic Shift Regulates Adult Neural Stem Cell Activity. Cell reports 20, 2144-2155
Knobloch M*, Braun SMG*, Zurkirchen L, von Schoultz C, Zamboni N, Kovacs WJ, Araùzo-Bravo MJ, Karalay O, Suter U, Machado R, Roccio M, Lutolf MP, Semenkovich CF, Jessberger S (2013) Metabolic control of adult neural stem cell activity by Fasn-dependent lipogenesis. Nature 493(7431):226-30
UZH press releases:
Lipid Metabolism Controls Brain Development
Lipid metabolism regulates the activity of adult neural stem cells
Regionalized forebrain organoids derived from human embryonic stem cells (e.g., Bowers et al., 2020 Cell Stem Cell). Credit: Daniel Gonzalez-Bohorquez, HiFo, UZH